Driven by the pandemic, the biotech industry turbocharged
29 May 2020 _ News

The outperformance of the nasdaq and the rebound of pharus sicav biotech shares confirm that the biotech sector has moved to turbo mode, also thanks to the coronavirus pandemic.
The pandemic has inevitably highlighted the strengths and weaknesses of the health system, highlighting what needs to be taken into account and what goes hand in hand with technological progress. And it has lifted, even more markedly, the veil from a sector with high growth potential: biotechnology, a candidate for winner for those who invest with an eye to the future.
The spread of a virus as unknown as it is contagious has necessarily also set a disruptive milestone in the medical and pharmacological sector, accelerating the process started in the 21st century in the application of new technologies to biology and the healthcare sector.
The benefits of biotechnology are not only quantifiable in terms of cost and time savings, but also in terms of human lives. It is characterized by being the most R&D-intensive sector, with more than double the traditional pharmaceutical industry and 5 times more than the market as a whole. This special status allows it to study and produce cures for rare diseases, orphan diseases, cardiovascular diseases, infections, viruses, neurological diseases (Alzheimer's disease, Parkinson's disease, etc.) and tumours.
In particular, Covid-19 has underlined the importance of big data: it is proving to be fundamental to tracking the spread of the virus - involving not only Covid patients, but also asymptomatic persons - through generic or routine tests; its use is also a great advantage for companies in charge of developing tests and producing devices for diagnosis. Further progress is being made with the spread of resources such as telemedicine and remote monitoring, which allow significant savings for the healthcare system and enable patients to avoid unnecessary hospital visits.
Robotics and virtual reality are also helping with "remote" treatment and surgery.
In the current scenario, where the further evolution of the coronavirus pandemic and its overcoming are likely to lead to a major review of global health risks and related health systems, the relevance of the biotechnology sector is expected to increase further. This has already been reflected in the fact that, since the beginning of the health scare, the biotechnology sector has started to outperform the Nasdaq:
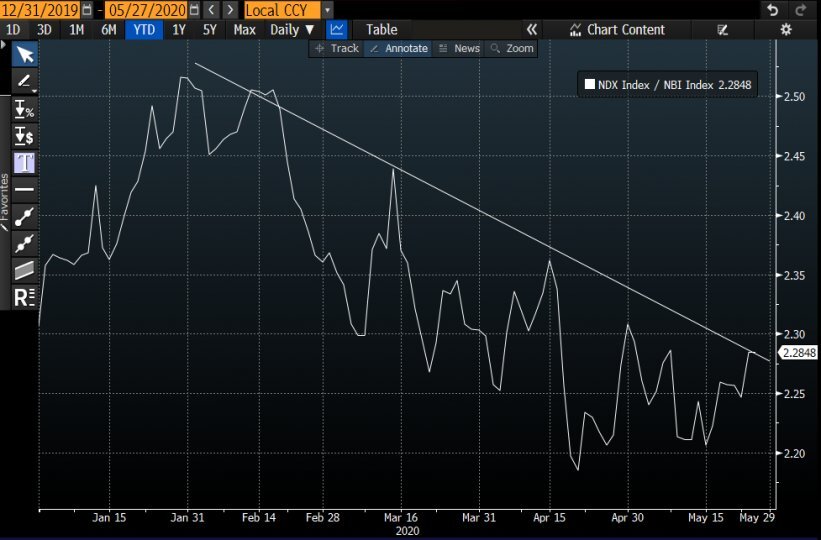
And in the rebound in share prices of many companies in the portfolio of Pharus Sicav Biotech since the first weeks of April, which has led to a performance of +4.47% since the beginning of the year (updated to 27 May 2020).
The biotech trend is not stopping; on the contrary, managers are always looking for new investments in biotech companies that combine clinical progress, improved quality of life and economic growth.
The coming months will focus mainly on the development of the Covid-19 vaccine and investment in research to support it:
- A high level of laboratory studies, their management and progress;
- The processes of acquisition and merger with the related licensing agreements;
- The proper management of developments from a regulatory perspective;
- A sound economic basis so that the most valuable active ingredients can compete in the markets and be the subject of careful analysis;
- Further development of products once promoted;
- A constant supply of medicines, avoiding possible interruptions in production.
But how is it possible that no laboratory in the world has managed to produce a vaccine yet? The answer is as simple as it is complex, and you have to start from the definition of a "classic" vaccine: to obtain a vaccine, minimal parts of the genetic material of the virus are introduced into human cells - as if the human body were in contact with a "weak" version of the virus - so that the immune system can start from the DNA or RNA segments of the foreign body and develop the necessary antibodies. These types of vaccines require a lower cost to develop and a shorter time, and are also easier to modify for a better immune defence - so researchers now have very little to start with at the moment.
The most reasonable expectation of an effective vaccine against Covid-19, available to everyone worldwide, is at least 2-3 years, which could be reduced to 18 months in the most optimistic case. This is an "unprecedented" period of time compared to the past, thanks in part to the enormous developments in biotechnology to date.
Information message - The information in this message is produced for information purposes only and therefore does not qualify as offer or recommendation or solicitation to buy or sell securities or financial instruments in general, financial products or services or investment, nor an exhortation to carry out transactions related to a specific financial instrument.
The contents of this informative message are the result of the free interpretation, evaluation and appreciation of Pharus Asset Management SA and constitute simple food for thought.
Any information and data indicated have a purely informative purpose and do not in any way represent an investment advisory service: the resulting operational decisions are to be considered taken by the user in full autonomy and at his own exclusive risk.
Pharus Asset Management SA dedicates the utmost attention and precision to the information contained in this message; nevertheless, no liability shall be accepted for errors, omissions, inaccuracies or manipulations by third parties on what is materially processed capable of affecting the correctness of the information provided and the reliability of the same, as well as for any result obtained using the said information.
It is not permitted to copy, alter, distribute, publish or use these contents on other sites for commercial use without the specific authorization of Pharus Asset Management SA.
The contents of this informative message are the result of the free interpretation, evaluation and appreciation of Pharus Asset Management SA and constitute simple food for thought.
Any information and data indicated have a purely informative purpose and do not in any way represent an investment advisory service: the resulting operational decisions are to be considered taken by the user in full autonomy and at his own exclusive risk.
Pharus Asset Management SA dedicates the utmost attention and precision to the information contained in this message; nevertheless, no liability shall be accepted for errors, omissions, inaccuracies or manipulations by third parties on what is materially processed capable of affecting the correctness of the information provided and the reliability of the same, as well as for any result obtained using the said information.
It is not permitted to copy, alter, distribute, publish or use these contents on other sites for commercial use without the specific authorization of Pharus Asset Management SA.
RELATED CONTENTS


